Phylogenetic Trees¶
Contents
Overview¶
Phylogenetic trees are the result of most evolutionary analyses. They represent the evolutionary relationships among a set of species or, in molecular biology, a set of homologous sequences.
The PhyloTree class is an extension of the base Tree
object, providing a appropriate way to deal with phylogenetic trees.
Thus, while leaves are considered to represent species (or sequences
from a given species genome), internal nodes are considered ancestral
nodes. A direct consequence of this is, for instance, that every split
in the tree will represent a speciation or duplication event.
Linking Phylogenetic Trees with Multiple Sequence Alignments¶
PhyloTree instances allow molecular phylogenies to be linked
to the Multiple Sequence Alignments (MSA). To associate a MSA with a
phylogenetic tree you can use the PhyloNode.link_to_alignment()
method. You can use the alg_format argument to specify its
format (See SeqGroup documentation for available formats)
Given that Fasta format are not only applicable for MSA but also for Unaligned Sequences, you may also associate sequences of different lengths with tree nodes.
from ete2 import PhyloTree
fasta_txt = """
>seqA
MAEIPDETIQQFMALT---HNIAVQYLSEFGDLNEALNSYYASQTDDIKDRREEAH
>seqB
MAEIPDATIQQFMALTNVSHNIAVQY--EFGDLNEALNSYYAYQTDDQKDRREEAH
>seqC
MAEIPDATIQ---ALTNVSHNIAVQYLSEFGDLNEALNSYYASQTDDQPDRREEAH
>seqD
MAEAPDETIQQFMALTNVSHNIAVQYLSEFGDLNEAL--------------REEAH
"""
# Load a tree and link it to an alignment.
t = PhyloTree("(((seqA,seqB),seqC),seqD);")
t.link_to_alignment(alignment=fasta_txt, alg_format="fasta")
The same could be done at the same time the tree is being loaded, by
using the alignment and alg_format arguments of
PhyloTree.
# Load a tree and link it to an alignment.
t = PhyloTree("(((seqA,seqB),seqC),seqD);", alignment=fasta_txt, alg_format="fasta")
As currently implemented, sequence linking process is not strict,
which means that a perfect match between all node names and sequences
names is not required. Thus, if only one match is found between
sequences names within the MSA file and tree node names, only one tree
node will contain an associated sequence. Also, it is important to
note that sequence linking is not limited to terminal nodes. If
internal nodes are named, and such names find a match within the
provided MSA file, their corresponding sequences will be also loaded
into the tree structure. Once a MSA is linked, sequences will be
available for every tree node through its node.sequence
attribute.
from ete2 import PhyloTree
fasta_txt = """
>seqA
MAEIPDETIQQFMALT---HNIAVQYLSEFGDLNEALNSYYASQTDDIKDRREEAH
>seqB
MAEIPDATIQQFMALTNVSHNIAVQY--EFGDLNEALNSYYAYQTDDQKDRREEAH
>seqC
MAEIPDATIQ---ALTNVSHNIAVQYLSEFGDLNEALNSYYASQTDDQPDRREEAH
>seqD
MAEAPDETIQQFMALTNVSHNIAVQYLSEFGDLNEAL--------------REEAH
"""
iphylip_txt = """
4 76
seqA MAEIPDETIQ QFMALT---H NIAVQYLSEF GDLNEALNSY YASQTDDIKD RREEAHQFMA
seqB MAEIPDATIQ QFMALTNVSH NIAVQY--EF GDLNEALNSY YAYQTDDQKD RREEAHQFMA
seqC MAEIPDATIQ ---ALTNVSH NIAVQYLSEF GDLNEALNSY YASQTDDQPD RREEAHQFMA
seqD MAEAPDETIQ QFMALTNVSH NIAVQYLSEF GDLNEAL--- ---------- -REEAHQ---
LTNVSHQFMA LTNVSH
LTNVSH---- ------
LTNVSH---- ------
-------FMA LTNVSH
"""
# Load a tree and link it to an alignment. As usual, 'alignment' can
# be the path to a file or data in text format.
t = PhyloTree("(((seqA,seqB),seqC),seqD);", alignment=fasta_txt, alg_format="fasta")
#We can now access the sequence of every leaf node
print "These are the nodes and its sequences:"
for leaf in t.iter_leaves():
print leaf.name, leaf.sequence
#seqD MAEAPDETIQQFMALTNVSHNIAVQYLSEFGDLNEAL--------------REEAH
#seqC MAEIPDATIQ---ALTNVSHNIAVQYLSEFGDLNEALNSYYASQTDDQPDRREEAH
#seqA MAEIPDETIQQFMALT---HNIAVQYLSEFGDLNEALNSYYASQTDDIKDRREEAH
#seqB MAEIPDATIQQFMALTNVSHNIAVQY--EFGDLNEALNSYYAYQTDDQKDRREEAH
#
# The associated alignment can be changed at any time
t.link_to_alignment(alignment=iphylip_txt, alg_format="iphylip")
# Let's check that sequences have changed
print "These are the nodes and its re-linked sequences:"
for leaf in t.iter_leaves():
print leaf.name, leaf.sequence
#seqD MAEAPDETIQQFMALTNVSHNIAVQYLSEFGDLNEAL--------------REEAHQ----------FMALTNVSH
#seqC MAEIPDATIQ---ALTNVSHNIAVQYLSEFGDLNEALNSYYASQTDDQPDRREEAHQFMALTNVSH----------
#seqA MAEIPDETIQQFMALT---HNIAVQYLSEFGDLNEALNSYYASQTDDIKDRREEAHQFMALTNVSHQFMALTNVSH
#seqB MAEIPDATIQQFMALTNVSHNIAVQY--EFGDLNEALNSYYAYQTDDQKDRREEAHQFMALTNVSH----------
#
# The sequence attribute is considered as node feature, so you can
# even include sequences in your extended newick format!
print t.write(features=["sequence"], format=9)
#
#
# (((seqA[&&NHX:sequence=MAEIPDETIQQFMALT---HNIAVQYLSEFGDLNEALNSYYASQTDDIKDRREEAHQF
# MALTNVSHQFMALTNVSH],seqB[&&NHX:sequence=MAEIPDATIQQFMALTNVSHNIAVQY--EFGDLNEALNSY
# YAYQTDDQKDRREEAHQFMALTNVSH----------]),seqC[&&NHX:sequence=MAEIPDATIQ---ALTNVSHNIA
# VQYLSEFGDLNEALNSYYASQTDDQPDRREEAHQFMALTNVSH----------]),seqD[&&NHX:sequence=MAEAPD
# ETIQQFMALTNVSHNIAVQYLSEFGDLNEAL--------------REEAHQ----------FMALTNVSH]);
#
# And yes, you can save this newick text and reload it into a PhyloTree instance.
sametree = PhyloTree(t.write(features=["sequence"]))
print "Recovered tree with sequence features:"
print sametree
#
# /-seqA
# /--------|
# /--------| \-seqB
# | |
#---------| \-seqC
# |
# \-seqD
#
print "seqA sequence:", (t&"seqA").sequence
# MAEIPDETIQQFMALT---HNIAVQYLSEFGDLNEALNSYYASQTDDIKDRREEAHQFMALTNVSHQFMALTNVSH
Visualization of phylogenetic trees¶
PhyloTree instances can benefit from all the features of the programmable drawing engine. However, a built-in phylogenetic layout is provided for convenience.
All PhyloTree instances are, by default, attached to such layout for tree visualization, thus allowing for in-place alignment visualization and evolutionary events labeling.
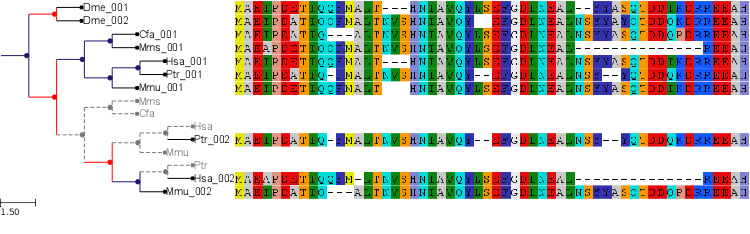
from ete2 import PhyloTree, TreeStyle
alg = """
>Dme_001
MAEIPDETIQQFMALT---HNIAVQYLSEFGDLNEAL--YYASQTDDIKDRREEAH
>Dme_002
MAEIPDATIQQFMALTNVSHNIAVQY--EFGDLNEALNSYYAYQTDDQKDRREEAH
>Cfa_001
MAEIPDATIQ---ALTNVSHNIAVQYLSEFGDLNEALNSYYASQTDDQPDRREEAH
>Mms_001
MAEAPDETIQQFMALTNVSHNIAVQYLSEFGDLNEAL--------------REEAH
>Hsa_001
MAEIPDETIQQFMALT---HNIAVQYLSEFGDLNEALNSYYASQTDDIKDRREEAH
>Ptr_002
MAEIPDATIQ-FMALTNVSHNIAVQY--EFGDLNEALNSY--YQTDDQKDRREEAH
>Mmu_002
MAEIPDATIQ---ALTNVSHNIAVQYLSEFGDLNEALNSYYASQTDDQPDRREEAH
>Hsa_002
MAEAPDETIQQFM-LTNVSHNIAVQYLSEFGDLNEAL--------------REEAH
>Mmu_001
MAEIPDETIQQFMALT---HNIAVQYLSEFGDLNEALNSYYASQTDDIKDRREEAH
>Ptr_001
MAEIPDATIQ-FMALTNVSHNIAVQY--EFGDLNEALNSY--YQTDDQKDRREEAH
>Mmu_001
MAEIPDATIQ---ALTNVSHNIAVQYLSEFGDLNEALNSYYASQTDDQPDRREEAH
"""
def get_example_tree():
# Performs a tree reconciliation analysis
gene_tree_nw = '((Dme_001,Dme_002),(((Cfa_001,Mms_001),((Hsa_001,Ptr_001),Mmu_001)),(Ptr_002,(Hsa_002,Mmu_002))));'
species_tree_nw = "((((Hsa, Ptr), Mmu), (Mms, Cfa)), Dme);"
genetree = PhyloTree(gene_tree_nw)
sptree = PhyloTree(species_tree_nw)
recon_tree, events = genetree.reconcile(sptree)
recon_tree.link_to_alignment(alg)
return recon_tree, TreeStyle()
if __name__ == "__main__":
# Visualize the reconciled tree
t, ts = get_example_tree()
t.show(tree_style=ts)
#recon_tree.render("phylotree.png", w=750)
Adding taxonomic information¶
PhyloTree instances allow to deal with leaf names and species
names separately. This is useful when working with molecular
phylogenies, in which node names usually represent sequence
identifiers. Species names will be stored in the
PhyloNode.species attribute of each leaf node. The method
PhyloNode.get_species() can be used obtain the set of species
names found under a given internal node (speciation or duplication
event). Often, sequence names do contain species information as a
part of the name, and ETE can parse this information automatically.
There are three ways to establish the species of the different tree nodes:
- Default: The three first letters of node’s name represent the species
- The species code of each node is dynamically created based on node’s name
- The species code of each node is manually set.
Automatic control of species info¶
from ete2 import PhyloTree
# Reads a phylogenetic tree (using default species name encoding)
t = PhyloTree("(((Hsa_001,Ptr_001),(Cfa_001,Mms_001)),(Dme_001,Dme_002));")
# /-Hsa_001
# /--------|
# | \-Ptr_001
# /--------|
# | | /-Cfa_001
# | \--------|
#---------| \-Mms_001
# |
# | /-Dme_001
# \--------|
# \-Dme_002
#
# Prints current leaf names and species codes
print "Deafult mode:"
for n in t.get_leaves():
print "node:", n.name, "Species name:", n.species
# node: Dme_001 Species name: Dme
# node: Dme_002 Species name: Dme
# node: Hsa_001 Species name: Hsa
# node: Ptr_001 Species name: Ptr
# node: Cfa_001 Species name: Cfa
# node: Mms_001 Species name: Mms
Automatic (and custom) control of the species info¶
The default behavior can be changed by using the
PhyloNode.set_species_naming_function() method or by using the
sp_naming_function argument of the PhyloTree class.
Note that, using the sp_naming_function argument, the whole
tree structure will be initialized to use the provided parsing
function to obtain species name
information. PhyloNode.set_species_naming_function() (present in
all tree nodes) can be used to change the behavior in a previously
loaded tree, or to set different parsing function to different parts
of the tree.
from ete2 import PhyloTree
# Reads a phylogenetic tree
t = PhyloTree("(((Hsa_001,Ptr_001),(Cfa_001,Mms_001)),(Dme_001,Dme_002));")
# Let's use our own leaf name parsing function to obtain species
# names. All we need to do is create a python function that takes
# node's name as argument and return its corresponding species name.
def get_species_name(node_name_string):
# Species code is the first part of leaf name (separated by an
# underscore character)
spcode = node_name_string.split("_")[0]
# We could even translate the code to complete names
code2name = {
"Dme":"Drosophila melanogaster",
"Hsa":"Homo sapiens",
"Ptr":"Pan troglodytes",
"Mms":"Mus musculus",
"Cfa":"Canis familiaris"
}
return code2name[spcode]
# Now, let's ask the tree to use our custom species naming function
t.set_species_naming_function(get_species_name)
print "Custom mode:"
for n in t.get_leaves():
print "node:", n.name, "Species name:", n.species
# node: Dme_001 Species name: Drosophila melanogaster
# node: Dme_002 Species name: Drosophila melanogaster
# node: Hsa_001 Species name: Homo sapiens
# node: Ptr_001 Species name: Pan troglodytes
# node: Cfa_001 Species name: Canis familiaris
# node: Mms_001 Species name: Mus musculus
Manual control of the species info¶
To disable the automatic generation of species names based on node
names, a None value can be passed to the
PhyloNode.set_species_naming_function() function. From then on,
species attribute will not be automatically updated based on the name
of nodes and it could be controlled manually.
from ete2 import PhyloTree
# Reads a phylogenetic tree
t = PhyloTree("(((Hsa_001,Ptr_001),(Cfa_001,Mms_001)),(Dme_001,Dme_002));")
# Of course, you can disable the automatic generation of species
# names. To do so, you can set the species naming function to
# None. This is useful to set the species names manually or for
# reading them from a newick file. Other wise, species attribute would
# be overwriten
mynewick = """
(((Hsa_001[&&NHX:species=Human],Ptr_001[&&NHX:species=Chimp]),
(Cfa_001[&&NHX:species=Dog],Mms_001[&&NHX:species=Mouse])),
(Dme_001[&&NHX:species=Fly],Dme_002[&&NHX:species=Fly]));
"""
t = PhyloTree(mynewick, sp_naming_function=None)
print "Disabled mode (manual set)"
for n in t.get_leaves():
print "node:", n.name, "Species name:", n.species
# node: Dme_001 Species name: Fly
# node: Dme_002 Species name: Fly
# node: Hsa_001 Species name: Human
# node: Ptr_001 Species name: Chimp
# node: Cfa_001 Species name: Dog
# node: Mms_001 Species name: Mouse
Full Example: Species aware trees.
Detecting evolutionary events¶
There are several ways to automatically detect duplication and speciation nodes. ETE provides two methodologies: One implements the algorithm described in Huerta-Cepas (2007) and is based on the species overlap (SO) between partitions and thus does not depend on the availability of a species tree. The second, which requires the comparison between the gene tree and a previously defined species tree, implements a strict tree reconciliation algorithm (Page and Charleston, 1997). By detecting evolutionary events, orthology and paralogy relationships among sequences can also be inferred. Find a comparison of both methods in Marcet-Houben and Gabaldon (2009).
Species Overlap (SO) algorithm¶
In order to apply the SO algorithm, you can use the
PhyloNode.get_descendant_evol_events() method (it will detect
all evolutionary events under the current node) or the
PhyloNode.get_my_evol_events() method (it will detect only the
evolutionary events in which current node, a leaf, is involved).
By default the species overlap score (SOS) threshold is set to
0.0, which means that a single species in common between two node
branches will rise a duplication event. This has been shown to perform
the best with real data, however you can adjust the threshold using
the sos_thr argument present in both methods.
from ete2 import PhyloTree
# Loads an example tree
nw = """
((Dme_001,Dme_002),(((Cfa_001,Mms_001),((Hsa_001,Ptr_001),Mmu_001)),
(Ptr_002,(Hsa_002,Mmu_002))));
"""
t = PhyloTree(nw)
print t
# /-Dme_001
# /--------|
# | \-Dme_002
# |
# | /-Cfa_001
# | /--------|
#---------| | \-Mms_001
# | /--------|
# | | | /-Hsa_001
# | | | /--------|
# | | \--------| \-Ptr_001
# \--------| |
# | \-Mmu_001
# |
# | /-Ptr_002
# \--------|
# | /-Hsa_002
# \--------|
# \-Mmu_002
#
# To obtain all the evolutionary events involving a given leaf node we
# use get_my_evol_events method
matches = t.search_nodes(name="Hsa_001")
human_seq = matches[0]
# Obtains its evolutionary events
events = human_seq.get_my_evol_events()
# Print its orthology and paralogy relationships
print "Events detected that involve Hsa_001:"
for ev in events:
if ev.etype == "S":
print ' ORTHOLOGY RELATIONSHIP:', ','.join(ev.in_seqs), "<====>", ','.join(ev.out_seqs)
elif ev.etype == "D":
print ' PARALOGY RELATIONSHIP:', ','.join(ev.in_seqs), "<====>", ','.join(ev.out_seqs)
# Alternatively, you can scan the whole tree topology
events = t.get_descendant_evol_events()
# Print its orthology and paralogy relationships
print "Events detected from the root of the tree"
for ev in events:
if ev.etype == "S":
print ' ORTHOLOGY RELATIONSHIP:', ','.join(ev.in_seqs), "<====>", ','.join(ev.out_seqs)
elif ev.etype == "D":
print ' PARALOGY RELATIONSHIP:', ','.join(ev.in_seqs), "<====>", ','.join(ev.out_seqs)
# If we are only interested in the orthology and paralogy relationship
# among a given set of species, we can filter the list of sequences
#
# fseqs is a function that, given a list of sequences, returns only
# those from human and mouse
fseqs = lambda slist: [s for s in slist if s.startswith("Hsa") or s.startswith("Mms")]
print "Paralogy relationships among human and mouse"
for ev in events:
if ev.etype == "D":
# Prints paralogy relationships considering only human and
# mouse. Some duplication event may not involve such species,
# so they will be empty
print ' PARALOGY RELATIONSHIP:', \
','.join(fseqs(ev.in_seqs)), \
"<====>",\
','.join(fseqs(ev.out_seqs))
# Note that besides the list of events returned, the detection
# algorithm has labeled the tree nodes according with the
# predictions. We can use such lables as normal node features.
dups = t.search_nodes(evoltype="D") # Return all duplication nodes
Tree reconciliation algorithm¶
Tree reconciliation algorithm uses a predefined species tree to infer all the necessary genes losses that explain a given gene tree topology. Consequently, duplication and separation nodes will strictly follow the species tree topology.
To perform a tree reconciliation analysis over a given node in a
molecular phylogeny you can use the PhyloNode.reconcile()
method, which requires a species PhyloTree as its first
argument. Leaf node names in the the species are expected to be the
same species codes in the gene tree (see
taxonomic_info). All species codes present in the
gene tree should appear in the species tree.
As a result, the PhyloNode.reconcile() method will label the
original gene tree nodes as duplication or speciation, will return the
list of inferred events, and will return a new reconcilied tree
(PhyloTree instance), in which inferred gene losses are
present and labeled.
from ete2 import PhyloTree
# Loads a gene tree and its corresponding species tree. Note that
# species names in sptree are the 3 firs letters of leaf nodes in
# genetree.
gene_tree_nw = '((Dme_001,Dme_002),(((Cfa_001,Mms_001),((Hsa_001,Ptr_001),Mmu_001)),(Ptr_002,(Hsa_002,Mmu_002))));'
species_tree_nw = "((((Hsa, Ptr), Mmu), (Mms, Cfa)), Dme);"
genetree = PhyloTree(gene_tree_nw)
sptree = PhyloTree(species_tree_nw)
print genetree
# /-Dme_001
# /--------|
# | \-Dme_002
# |
# | /-Cfa_001
# | /--------|
#---------| | \-Mms_001
# | /--------|
# | | | /-Hsa_001
# | | | /--------|
# | | \--------| \-Ptr_001
# \--------| |
# | \-Mmu_001
# |
# | /-Ptr_002
# \--------|
# | /-Hsa_002
# \--------|
# \-Mmu_002
#
# Let's reconcile our genetree with the species tree
recon_tree, events = genetree.reconcile(sptree)
# a new "reconcilied tree" is returned. As well as the list of
# inferred events.
print "Orthology and Paralogy relationships:"
for ev in events:
if ev.etype == "S":
print 'ORTHOLOGY RELATIONSHIP:', ','.join(ev.inparalogs), "<====>", ','.join(ev.orthologs)
elif ev.etype == "D":
print 'PARALOGY RELATIONSHIP:', ','.join(ev.inparalogs), "<====>", ','.join(ev.outparalogs)
# And we can explore the resulting reconciled tree
print recon_tree
# You will notice how the reconcilied tree is the same as the gene
# tree with some added branches. They are inferred gene losses.
#
#
# /-Dme_001
# /--------|
# | \-Dme_002
# |
# | /-Cfa_001
# | /--------|
# | | \-Mms_001
#---------| /--------|
# | | | /-Hsa_001
# | | | /--------|
# | | \--------| \-Ptr_001
# | | |
# | | \-Mmu_001
# \--------|
# | /-Mms
# | /--------|
# | | \-Cfa
# | |
# | | /-Hsa
# \--------| /--------|
# | /--------| \-Ptr_002
# | | |
# | | \-Mmu
# \--------|
# | /-Ptr
# | /--------|
# \--------| \-Hsa_002
# |
# \-Mmu_002
#
# And we can visualize the trees using the default phylogeny
# visualization layout
genetree.show()
recon_tree.show()
A closer look to the evolutionary event object¶
Both methods, species overlap and tree reconciliation, can be used to
label each tree node as a duplication or speciation event. Thus, the
PhyloNode.evoltype attribute of every node will be set to one
of the following states: D (Duplication), S (Speciation) or
L gene loss.
Additionally, a list of all the detected events is returned. Each
event is a python object of type phylo.EvolEvent, containing
some basic information about each event ( etype,
in_seqs, out_seqs, node):
If an event represents a duplication, in_seqs are all
paralogous to out_seqs. Similarly, if an event represents a
speciation, in_seqs are all orthologous to out_seqs.
Relative dating phylogenetic nodes¶
In molecular phylogeny, nodes can be interpreted as evolutionary events. Therefore, they represent duplication or speciation events. In the case of gene duplication events, nodes can also be assigned to a certain point in a relative temporal scale. In other words, you can obtain a relative dating of all the duplication events detected.
Although absolute dating is always preferred and more precise, topological dating provides a faster approach to compare the relative age of paralogous sequences (read this for a comparison with other methods, such as the use of synonymous substitution rates as a proxy to the divergence time).
Some applications of topological dating can be found in Huerta-Cepas et al, 2007 or, more recently, in Huerta-Cepas et al, 2011 or Kalinka et al, 2001.
Implementation¶
The aim of relative dating is to establish a gradient of ages among sequences. For this, a reference species needs to be fixed, so the gradient of ages will be referred to that referent point.
Thus, if our reference species is Human, we could establish the following gradient of species:
- (1) Human -> (2) Other Primates -> (3) Mammals -> (4) Vertebrates
So, nodes in a tree can be assigned to one of the above categories depending on the sequences grouped. For instance:
- A node with only human sequences will be mapped to (1).
- A node with human and orangutan sequences will be mapped to (2)
- A node with human a fish sequences will be mapped to (4)
This simple calculation can be done automatically by encoding the gradient of species ages as Python dictionary.
relative_dist = {
"human": 0, # human
"chimp": 1, # Primates non human
"rat": 2, # Mammals non primates
"mouse": 2, # Mammals non primates
"fish": 3 # Vertebrates non mammals
}
Once done, ETE can check the relative age of any tree node. The
PhyloNode.get_age() method can be used to that purpose.
For example, let’s consider the following gene tree:
# /-humanA
# /---|
# | \-chimpA
# /Dup1
# | | /-humanB
# /---| \---|
# | | \-chimpB
# /---| |
# | | \-mouseA
# | |
# | \-fish
#-Dup3
# | /-humanC
# | /---|
# | /---| \-chimpC
# | | |
# \Dup2 \-humanD
# |
# | /-ratC
# \---|
# \-mouseC
the expected node dating would be:
- Dup1 will be assigned to primates (most distant species is chimp).
Dup1.get_age(relative_distances)will return 1- Dup2 will be assigned to mammals [2] (most distant species are rat and mouse).
Dup2.get_age(relative_distances)will return 2- Dup3 will be assigned to mammals [3] (most distant species is fish).
Dup3.get_age(relative_distances)will return 3
from ete2 import PhyloTree
# Creates a gene phylogeny with several duplication events at
# different levels. Note that we are using the default method for
# detecting the species code of leaves (three first lettes in the node
# name are considered the species code).
nw = """
((Dme_001,Dme_002),(((Cfa_001,Mms_001),((((Hsa_001,Hsa_003),Ptr_001)
,Mmu_001),((Hsa_004,Ptr_004),Mmu_004))),(Ptr_002,(Hsa_002,Mmu_002))));
"""
t = PhyloTree(nw)
print "Original tree:",
print t
#
# /-Dme_001
# /--------|
# | \-Dme_002
# |
# | /-Cfa_001
# | /--------|
# | | \-Mms_001
# | |
#--| | /-Hsa_001
# | | /--------|
# | /--------| /--------| \-Hsa_003
# | | | | |
# | | | /--------| \-Ptr_001
# | | | | |
# | | | | \-Mmu_001
# | | \--------|
# \--------| | /-Hsa_004
# | | /--------|
# | \--------| \-Ptr_004
# | |
# | \-Mmu_004
# |
# | /-Ptr_002
# \--------|
# | /-Hsa_002
# \--------|
# \-Mmu_002
# Create a dictionary with relative ages for the species present in
# the phylogenetic tree. Note that ages are only relative numbers to
# define which species are older, and that different species can
# belong to the same age.
species2age = {
'Hsa': 1, # Homo sapiens (Hominids)
'Ptr': 2, # P. troglodytes (primates)
'Mmu': 2, # Macaca mulata (primates)
'Mms': 3, # Mus musculus (mammals)
'Cfa': 3, # Canis familiaris (mammals)
'Dme': 4 # Drosophila melanogaster (metazoa)
}
# We can translate each number to its correspondig taxonomic number
age2name = {
1:"hominids",
2:"primates",
3:"mammals",
4:"metazoa"
}
event1= t.get_common_ancestor("Hsa_001", "Hsa_004")
event2=t.get_common_ancestor("Hsa_001", "Hsa_002")
print
print "The duplication event leading to the human sequences Hsa_001 and "+\
"Hsa_004 is dated at: ", age2name[event1.get_age(species2age)]
print "The duplication event leading to the human sequences Hsa_001 and "+\
"Hsa_002 is dated at: ", age2name[event2.get_age(species2age)]
# The duplication event leading to the human sequences Hsa_001 and Hsa_004
# is dated at: primates
#
# The duplication event leading to the human sequences Hsa_001 and Hsa_002
# is dated at: mammals
Warning
Note that relative distances will vary depending on your reference species.
Automatic rooting (outgroup detection)¶
Two methods are provided to assist in the automatic rooting of phylogenetic trees. Since tree nodes contain relative age information (based on the species code autodetection), the same relative age dictionaries can be used to detect the farthest and oldest node in a tree to given sequences.
PhyloNode.get_farthest_oldest_node() and
PhyloNode.get_farthest_oldest_leaf() can be used for that
purpose.
Working with duplicated gene families¶
Treeko (splitting gene trees into species trees)¶
Comparisons between tree topologies provide important information for many evolutionary studies. Treeko (Marcet and Gabaldon, 2011 ) is a novel method that allows the comparison of any two tree topologies, even those with missing leaves and duplications. This is important in genome-wide analysis since many trees do not have exact leaf pairings and therefore most tree comparison methods are rendered useless.
Although Treeko is available as a standalone package, it uses ETE to generate all possible species tree topologies within a duplicated gene family tree.
Thus, the ETE method PhyloNode.get_speciation_trees() is
expected to provide the core functionality required to perform a
Treeko analysis. When used, the method will return a list of all
possible species trees observed after combining the different
non-duplicated subparts under a gene family tree node.
Duplication events will be automatically identified using the species
overlap algorithm described within this manual. However, duplication
nodes can be manually labeled and used by disabling the
autodetect_duplication flag.
Because of the combinatorial background of the Treeko method, the number of speciation trees generated by this function may vary enormously (ranging from few hundreds to tens of thousands topologies).
Here is a basic example on how to use it:
from ete2 import PhyloTree
t = PhyloTree("((((Human_1, Chimp_1), (Human_2, (Chimp_2, Chimp_3))), ((Fish_1, (Human_3, Fish_3)), Yeast_2)), Yeast_1);")
t.set_species_naming_function(lambda node: node.name.split("_")[0] )
print t.get_ascii(attributes=["name", "species"], show_internal=False )
# /-Human_1, Human
# /-|
# | \-Chimp_1, Chimp
# /-|
# | | /-Human_2, Human
# | \-|
# | | /-Chimp_2, Chimp
# | \-|
# /-| \-Chimp_3, Chimp
# | |
# | | /-Fish_1, Fish
# | | /-|
# | | | | /-Human_3, Human
# --| \-| \-|
# | | \-Fish_3, Fish
# | |
# | \-Yeast_2, Yeast
# |
# \-Yeast_1, Yeast
# We obtain a list of species trees inferred from the duplication
# events. Note that species specific duplications are ignored.
ntrees, ndups, sptrees = t.get_speciation_trees()
print "Found %d species trees and %d duplication nodes" %(ntrees, ndups)
for spt in sptrees:
print spt
# Found 5 species trees and 4 duplication nodes
#
# /-Human_1
# --|
# \-Chimp_1
#
# /-Human_2
# --|
# | /-Chimp_2
# \-|
# \-Chimp_3
#
# /-Fish_1
# --|
# \-Yeast_2
#
# /-Human_3
# /-|
# --| \-Fish_3
# |
# \-Yeast_2
#
# --Yeast_1
Note
For performance reasons, species trees are created without any link
to the original gene family tree, rather than the species name of
each node. However, the map_features attribute can be used
to keep certain attributes of the original tree into the generated
species trees.
Note
Although the efficiency of the method to generate all possible
trees has been significantly improved from ETE version 2.2,
creating thousands of new PhyloTree objects could affect
performance. The flag newick_only is now available to limit
the output to a newick string per generated tree, thus improving
the speed they can be processed or dumped into a file.
Splitting gene trees by duplication events¶
A much simpler approach to separate duplicates within the same gene
family tree is to split the topology by their duplication nodes. For
this, the method PhyloNode.split_by_dups() is provided.
from ete2 import PhyloTree
t = PhyloTree("((((Human_1, Chimp_1), (Human_2, (Chimp_2, Chimp_3))), ((Fish_1, (Human_3, Fish_3)), Yeast_2)), Yeast_1);")
t.set_species_naming_function(lambda node: node.name.split("_")[0] )
print t.get_ascii(attributes=["name", "species"], show_internal=False )
# /-Human_1, Human
# /-|
# | \-Chimp_1, Chimp
# /-|
# | | /-Human_2, Human
# | \-|
# | | /-Chimp_2, Chimp
# | \-|
# /-| \-Chimp_3, Chimp
# | |
# | | /-Fish_1, Fish
# | | /-|
# | | | | /-Human_3, Human
# --| \-| \-|
# | | \-Fish_3, Fish
# | |
# | \-Yeast_2, Yeast
# |
# \-Yeast_1, Yeast
# Again, species specific duplications are ignored
for node in t.split_by_dups():
print node
# /-Human_1
# --|
# \-Chimp_1
#
# /-Human_2
# --|
# | /-Chimp_2
# \-|
# \-Chimp_3
#
# --Yeast_2
#
# --Fish_1
#
# /-Human_3
# --|
# \-Fish_3
#
# --Yeast_1
Collapse species specific duplications¶
The method PhyloNode.collapse_lineage_specific_expansions()
method, which returns a pruned version of a tree, where nodes
representing lineage specific expansions are converted into a single
leaf node is also available.
From the previous examples, the lineage specific duplication of Chimp_1 and Chimp_2 could be easily collapsed into a single node.
from ete2 import PhyloTree
t = PhyloTree("((((Human_1, Chimp_1), (Human_2, (Chimp_2, Chimp_3))), ((Fish_1, (Human_3, Fish_3)), Yeast_2)), Yeast_1);")
t.set_species_naming_function(lambda node: node.name.split("_")[0] )
print t.get_ascii(attributes=["name", "species"], show_internal=False )
# /-Human_1, Human
# /-|
# | \-Chimp_1, Chimp
# /-|
# | | /-Human_2, Human
# | \-|
# | | /-Chimp_2, Chimp
# | \-|
# /-| \-Chimp_3, Chimp
# | |
# | | /-Fish_1, Fish
# | | /-|
# | | | | /-Human_3, Human
# --| \-| \-|
# | | \-Fish_3, Fish
# | |
# | \-Yeast_2, Yeast
# |
# \-Yeast_1, Yeast
t2 = t.collapse_lineage_specific_expansions()
print t2.get_ascii(attributes=["name", "species"], show_internal=False )
# /-Human_1, Human
# /-|
# | \-Chimp_1, Chimp
# /-|
# | | /-Human_2, Human
# | \-|
# | \-Chimp_2, Chimp ***
# /-|
# | | /-Fish_1, Fish
# | | /-|
# | | | | /-Human_3, Human
# --| \-| \-|
# | | \-Fish_3, Fish
# | |
# | \-Yeast_2, Yeast
# |
# \-Yeast_1, Yeast
